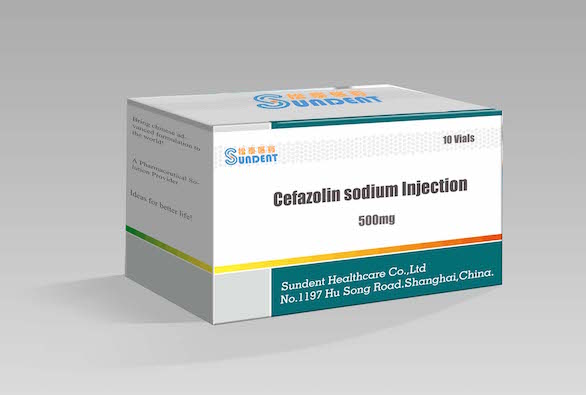

Cefazolin for Injection
-
Payment


-
Origin
China Mainland
-
Minimum Order
1
-
Packing
Pieces
- Contact Now Start Order
- Description
Product Detail
Cefazolin for injection
Instruction
Product Name:Cefazolin sodium for Injection
Nombre del Producto:Cefazolina sódica para solución inyectable
Specification: 500mg,1g
Package: 10viales/box, 50viales/box, 100viales/box
Standard: BP & USP & CP
Indications
It is indicated in the treatment of:
-Respiratory tract infections, acute and subacute bronchitis, bronchopneumonia, pneumo-pulmonary abscess, pharyngitis, laryngitis and tonsiiitis.
- Septicemia and subacute bacterial endocarditis.
- Cholangitis, cholecystitis, peritonitis, lymphangitis and lymphadenitis.
- Genitourinary tract infections.
-Osteomyelitis, arthiritis, folliculitis, furunculosis, atheroma, carbuncle, erysipelas, abscess, post-operative wound infections, wound infections, burns and scalds.
Dosage and administration
Cefazolin for Injection, USP may be administered either intramuscularly or intravenously
after constitution. In both cases, total daily dosages are the same.
Treatment should be continued in beta-hemolytic streptococcal infections for at least
10 days to minimize possible complications associated with the disease.
After reconstitution
1 - Mild-moderate Infections:
- Adults: 500 mg - 1 gm every 12 hours.
- Children: 25 - 50 mg/kg/day in 3 or 4 equally divided doses.
2 - Moderate - severe infections:
- Adults: 500 mg -1 gm every 6 - 8 hours.
- Children: up to 100 mg/kg/day in 3 or 4 equally divided doses.
Contra-indications
Sterile cefazolin sodium is contraindicated in patients with known allergy to the cephalosporin
group of antibiotics.
Warnings
Sterile cefazolin sodium should be used with caution in penicillin-allergic patients. There is
clinical evidence of partial cross-allergenicity of the penicillins and the cephalosporins. There
are instances of patients who have had reactions to both penicillins and cephalosporins (including
fatal anaphylaxis after parenteral use). Clinical and laboratory evidence of partial crossallergenicity
of the two drug classes exists.
Sterile cefazolin sodium should be administered cautiously and then only when absolutely
necessary to any patient who has demonstrated allergy, particularly to drugs. Immediate
emergency treatment with epinephrine is indicated for serious anaphylactoid reactions. As
indicated, oxygen, intravenous steroids, and airway management including intubation, should
also be employed.
There have been reports of pseudo membranous colitis with the use of cephalosporins. It is
therefore important to consider its diagnosis in patients who develop diarrhea in association with
antibiotic use.
Related Keywords: We are considered as one of the leading manufacturers and suppliers of cefazolin for injection. With the aid of our technologically advanced manufacturing section, we are able to offer customers cefazolin for injection products which are tested on various quality parameters in order to supply flawless range at the customers' end.
- Ceftriaxone for Injection 1 Pieces / (Min. Order)
- Clindamycin Injection 1 Pieces / (Min. Order)
- Ampicillin Sodium Injection 1 Pieces / (Min. Order)
- Fosfomycin Sodium for Injection 1 Pieces / (Min. Order)


 Favorites
Favorites